Who Should Avoid Fillers?
Get Your Dermal Filler Consultation with Dr. Laura Geige at It’s Me and You Clinic
Medical Conditions that May Require Cautious Use of Fillers
“Individuals with certain medical conditions or autoimmune disorders may need to exercise caution when using cosmetic fillers,” warns a leading dermatologist. “It’s essential to understand which conditions may require more careful consideration before proceeding with filler treatments.
Autimmune disorders, such as lupus, rheumatoid arthritis, and Hashimoto’s thyroiditis, can impact the body’s ability to heal and may increase the risk of complications from fillers,” explains a leading plastic surgeon. “For example, individuals with lupus are more prone to blood clots and scarring, which can be exacerbated by filler injections.”
“People with a history of keloid or hypertrophic scarring should also approach filler treatments with caution,” advises a board-certified dermatologist. “Fillers may cause the formation of new scars or the growth of existing ones, leading to raised, thickened, or discolored skin.
Autoimmune disorders that affect the nervous system, such as multiple sclerosis or peripheral neuropathy, can increase the risk of complications from fillers,” warns a neurologist. “Filler injections may cause numbness, tingling, or weakness in the treated area, which can be particularly problematic for individuals with existing nerve damage.
Those with bleeding disorders, such as hemophilia or von Willebrand disease, should also exercise caution when using fillers,” notes a hematologist. “Filler injections may cause prolonged bleeding or bruising at the treatment site, which can lead to serious complications.
“Individuals with a history of allergic reactions to previous filler treatments should avoid using fillers altogether until they have been properly assessed and cleared by their healthcare provider,” advises a leading allergist. “Even mild allergic reactions can lead to severe symptoms, including anaphylaxis.”
Moreover, people with certain genetic disorders, such as Marfan syndrome or Ehlers-Danlos syndrome, may be at increased risk of complications from fillers due to fragile skin and connective tissue,” explains a geneticist. “Filler treatments can cause blood vessels to rupture or skin to stretch beyond its normal limits, leading to serious consequences.
“It’s crucial for individuals with these conditions to consult with their healthcare provider before pursuing filler treatments,” emphasizes a leading plastic surgeon. “Together, they can weigh the potential benefits and risks of fillers and develop a personalized treatment plan that minimizes harm and optimizes results.”
Individuals with autoimmune disorders, such as *_Lupus_* or *_Rheumatoid Arthritis_*, may need to exercise caution when using fillers.
Autoimmune disorders occur when the body’s immune system mistakenly attacks healthy tissues, leading to inflammation and damage. In the case of *_Lupus_* and *_Rheumatoid Arthritis_*, this can result in chronic inflammation that affects multiple systems in the body.
Fillers, such as *_Hyaluronic Acid Fillers_* and *_Calcium Hydroxylapatite Fillers_*, are commonly used to restore lost volume and smooth out wrinkles. However, these substances can trigger an immune response in individuals with autoimmune disorders.
In people with *_Lupus_*, the use of fillers has been linked to increased risk of _**Seroma formation**_, which is a fluid collection that can occur at the injection site. This can lead to pain, swelling, and scarring.
Furthermore, individuals with *_Rheumatoid Arthritis_* may be more susceptible to complications from filler injections due to their underlying inflammatory condition. _**Osteoporosis***, a condition characterized by brittle bones, is also a concern, as fillers can cause bone fragmentation or implant failure in these patients.
Other autoimmune disorders that may require cautious use of fillers include *_Sjögren’s Syndrome_*, *_Hashimoto’s Thyroiditis_*, and *_Psoriasis_*. Individuals with these conditions should consult with their dermatologist or rheumatologist before undergoing filler treatments.
It is essential for healthcare professionals to assess each patient’s individual risk factors and medical history before administering fillers. This may involve discussing the potential benefits and risks of treatment, as well as alternative options for achieving desired results.
Some alternative approaches that may be recommended for individuals with autoimmune disorders include:
- Non-invasive treatments, such as *_Botox_* injections or _**Chemical Peels***, which can help reduce fine lines and wrinkles without the risk of an immune response.
- *Immunomodulatory therapies*, which can help regulate the immune system and reduce inflammation in autoimmune disorders.
- Dermal fillers made from biocompatible materials, such as *_Poly-L-Lactic Acid Fillers*** or *_Collagen Fillers***, which may be less likely to trigger an immune response than hyaluronic acid or calcium hydroxylapatite fillers.
Ultimately, the decision to use fillers should be made on a case-by-case basis, taking into account the individual’s unique medical history and risk factors. It is crucial for healthcare professionals to carefully weigh the potential benefits and risks of treatment before administering fillers to patients with autoimmune disorders.
If you have a history of autoimmune skin conditions, such as lupus or psoriasis, it is essential to exercise caution when considering cosmetic fillers. These conditions can cause inflammation and scarring in the skin, which may be exacerbated by the filler material.
Furthermore, some autoimmune skin conditions, like scleroderma, can lead to changes in skin texture and elasticity. In these cases, the use of fillers may not provide the desired results or may even worsen the underlying condition.
Autonomic nervous system disorders, such as Parkinson’s disease or multiple system atrophy, can also impact the distribution and absorption of fillers. This is because fillers are absorbed through nerve endings in the skin, which can lead to uneven or incomplete placement.
Individuals with neurodegenerative diseases may also be advised against using certain types of fillers, such as hyaluronic acid-based products. These diseases can cause changes in the brain’s ability to regulate pain and inflammation, which may lead to adverse reactions to these fillers.
Eczema or atopic dermatitis is another condition that may require cautious use of fillers. The skin in patients with eczema often has a compromised barrier function, making it more susceptible to irritation from filler materials.
Additionally, some patients with conditions like rosacea or erythromelalgia may need to be more careful when using fillers. These conditions cause inflammation and vasodilation in the skin, which can make it challenging to achieve optimal results with fillers.
It’s also worth noting that patients taking immunosuppressive medications, such as those used to treat rheumatoid arthritis or cancer, may be at higher risk for complications from filler use. These medications can suppress the immune system, making it more difficult for the body to reject foreign materials like fillers.
Finally, individuals with a history of skin cancers, such as squamous cell carcinoma or basal cell carcinoma, should consult their dermatologist before undergoing any cosmetic procedures that involve fillers. The presence of cancer can increase the risk of complications from filler use, and it’s essential to rule out any underlying conditions that may interact with the filler material.
Pregnancy and Breastfeeding
Pregnancy and breastfeeding can pose risks to the fetus or infant if certain medications are used, particularly those containing fillers. Fillers are often added to injectable medications to help maintain stability and extend shelf life.
Some common fillers include albumin, which is derived from human serum; gelatin, a protein found in animal products; and dextrose, a type of sugar. While generally considered safe for non-pregnant individuals, the use of these fillers during pregnancy or breastfeeding has raised concerns among healthcare providers.
Albumin, in particular, has been linked to an increased risk of blood clots, particularly in pregnant women with a history of clotting disorders. Additionally, some studies have suggested that albumin may cross the placental barrier, potentially exposing the fetus to toxic levels of heavy metals such as mercury and lead.
Gelatin, on the other hand, is derived from animal products and has been linked to an increased risk of adverse reactions in pregnant women, including allergic responses and anaphylaxis. Furthermore, gelatin can cause digestive issues in infants, particularly if ingested through breastfeeding or formula supplementation.
Dextrose is generally considered safe during pregnancy and breastfeeding, but high concentrations may be associated with an increased risk of hypoglycemia (low blood sugar) in the infant. This is particularly concerning for newborns who may not have fully developed their glucose regulation mechanisms.
The risk to the fetus or infant depends on various factors, including the type and dosage of the medication, the gestational age of the fetus, and the individual’s overall health status. Pregnant women should discuss their medication regimen with their healthcare provider, who can assess the potential risks and benefits of fillers in their specific situation.
Breastfeeding mothers may also want to consider the type of filler used in their medication. For example, some studies suggest that human serum albumin-based products may not be as safe during breastfeeding, as they may contain higher levels of heavy metals than bovine-derived albumin products.
To minimize risks, healthcare providers often recommend using medications with alternative fillers or opting for oral or topical formulations instead of injectables whenever possible. Pregnant women and breastfeeding mothers should work closely with their healthcare provider to ensure the safe use of medications containing fillers during pregnancy and lactation.
Ultimately, the decision to use a medication containing fillers during pregnancy or breastfeeding should be made on an individual basis, taking into account the potential risks and benefits. Healthcare providers can help patients weigh these factors and make informed decisions about their care.
Pregnancy and Breastfeeding are significant life milestones, requiring careful consideration of one’s health and wellbeing. During these periods, it is crucial to be mindful of the substances used in personal care products, such as fillers, to minimize potential risks.
Fillers, also known as excipients, are ingredients added to pharmaceuticals and personal care products to enhance their texture, stability, or shelf life. However, some fillers contain substances like parabens, formaldehyde, or mercury, which have raised concerns due to potential health risks.
Pregnancy Risks: During pregnancy, the body undergoes significant changes, and the fetus is vulnerable to environmental toxins. Exposure to parabens, a type of preservative commonly used in personal care products, has been linked to an increased risk of reproductive issues, such as birth defects and infertility. Formaldehyde, another common filler, has been shown to cause cancer and other health problems.
Breastfeeding Risks: Breast milk is the primary source of nutrition for infants, and exposure to toxins during pregnancy can affect breast milk composition and quality. Mercury, a potent neurotoxin, can accumulate in breast milk and pose risks to infant brain development and health. Even low levels of mercury exposure have been linked to cognitive and behavioral problems in children.
As a result, pregnant women are advised to avoid products containing parabens, formaldehyde, or mercury, including fillers like talc, silica, or calcium sulfate. Instead, opting for products labeled as paraben-free and mercury-free can help minimize exposure to these substances.
Furthermore, some personal care products may contain formaldehyde-releasing agents, such as quaternium-15 or DMDM hydantoin. These ingredients release formaldehyde over time and can accumulate in the body. Pregnant women are advised to choose products with alternative preservatives, like phenoxyethanol or benzyl alcohol.
Additionally, some breast milk alternatives, such as formulas or supplements, may contain fillers like mercury-contaminated fish oil or formaldehyde-releasing agents. Pregnant women and breastfeeding mothers should always check the ingredient labels and opt for products certified as mercury-free and paraben-free.
In conclusion, avoiding fillers containing parabens, formaldehyde, or mercury during pregnancy and breastfeeding is crucial to minimize potential health risks. By choosing products with safer ingredients and opting for certified mercury-free and paraben-free alternatives, pregnant women and breastfeeding mothers can help ensure a healthier environment for themselves and their infants.
The use of fillers in personal care products, such as lotions, creams, and soaps, has become increasingly popular in recent years. However, some of these fillers have been linked to reproductive toxicity and other health concerns.
When it comes to pregnancy and breastfeeding, it is essential to be aware of the potential risks associated with certain fillers. The European Chemicals Agency recommends avoiding fillers that contain known reproductive toxicants.
In this context, who should avoid fillers? While everyone’s body is different, some individuals may want to take extra precautions when using personal care products containing fillers during pregnancy and breastfeeding.
Women who are pregnant or breastfeeding should consider the following individuals:
- Those who have a history of reproductive issues or fertility problems
- Individuals with sensitivities or allergies to certain ingredients
- Mothers-to-be who work in industries where they are exposed to toxic chemicals on a daily basis (e.g. factory workers, healthcare professionals)
- Pregnant women with multiple pregnancies or those carrying twins, triplets, or other multiples
- Women breastfeeding for an extended period (i.e. over 6 months) and who have not yet returned to their pre-pregnancy fertility window
The following fillers are known reproductive toxicants and should be avoided during pregnancy and breastfeeding:
Fillers with known reproductive toxicants include:
- Para-Phenylene Diamine (PPD) – used in hair dye, soaps, and cosmetics
- 4-Methoxymethylphenyl Ketone (PMK) – a UV stabilizer used in personal care products
- Bisphenol A (BPA) – used in some plastics and resins
- Toluene-2,5-diamine (PTD) – used in hair dye and soaps
- Formaldehyde-releasing agents – used in hair products, adhesives, and other applications
- P-Phenyldimine (PPD) – used in soaps, cosmetics, and other personal care products
When shopping for personal care products, be sure to read labels carefully and look out for certifications like the European Chemicals Agency’s REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) mark. This symbol indicates that the product has been evaluated for reproductive toxicity and is safe for use during pregnancy and breastfeeding.
Additionally, choose products from manufacturers who prioritize sustainability, natural ingredients, and eco-friendliness. These options tend to be free from harsh chemicals, including those known reproductive toxicants.
In conclusion, while the risks associated with fillers are still a topic of debate, being aware of potential concerns and taking steps to minimize exposure can help ensure a healthier pregnancy and breastfeeding experience for mothers-to-be and their families.
Medical Conditions that Require Specialized Care
Arrange Your Dermal Filler Session with Dr. Laura Geige
The presence of a history of blood clots in an individual’s medical record is a crucial factor in determining whether they are suitable candidates for certain medical procedures, including those that involve the use of fillers.
Blood clots, also known as thrombosis, occur when a blood vessel becomes blocked by a clot of blood. This can be life-threatening and requires prompt medical attention. Certain conditions that increase the risk of developing blood clots include atrial fibrillation, deep vein thrombosis (DVT), pulmonary embolism (PE), and stroke.
Secure a Dermal Filler Consultation with Dr. Laura Geige Now
Individuals with a history of blood clots may require specialized care to prevent future clotting episodes. This can involve regular monitoring by healthcare providers, medication to prevent clot formation, and lifestyle modifications such as regular exercise and a healthy diet.
In the context of fillers, individuals with a history of blood clots should exercise extreme caution. Certain fillers, such as those made from hyaluronic acid or collagen, may increase the risk of blood clots forming at the injection site.
For example, patients who have had a previous clotting episode or are taking anticoagulant medications to prevent future clots should not receive fillers that contain these ingredients. Additionally, individuals with certain underlying medical conditions, such as cancer or inflammatory arthritis, may also require specialized care and caution when receiving fillers.
History of blood clots in the legs (deep vein thrombosis) can pose a risk for pulmonary embolism (blood clot in lungs), which may be life-threatening. Such patients should exercise extreme caution before getting fillers made from hyaluronic acid or collagen.
Additionally, individuals who have experienced blood clots in other parts of their body, such as the brain (stroke) or liver, should consult their healthcare provider carefully before undergoing a filler treatment. These conditions can indicate an underlying cardiovascular disease that may require specialized care when receiving fillers.
History of clotting disorders (such as Factor V Leiden thrombophilia) also carries increased risks for developing blood clots after getting fillers made from hyaluronic acid or collagen. Therefore, individuals with such a history should consult their healthcare provider carefully before undergoing filler treatments.
Certain medications, including anticoagulants and nonsteroidal anti-inflammatory drugs (NSAIDs), can increase the risk of bleeding when combined with fillers. Patients taking these medications should discuss this information with their healthcare provider before receiving a filler treatment to ensure safe administration.
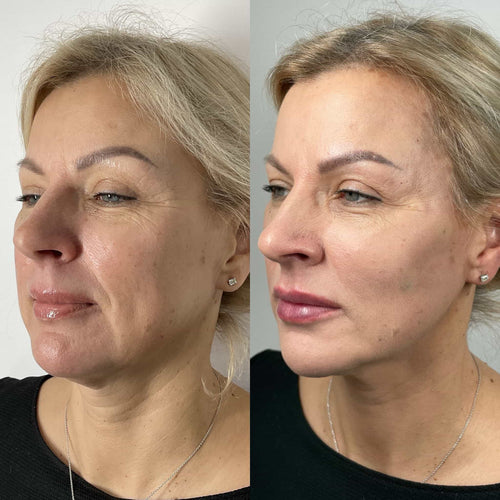
The use of dermal fillers has become increasingly popular over the past decade, with many individuals seeking to enhance the appearance of their facial features.
However, there are certain medical conditions that require specialized care, and individuals with a history of blood clots, such as deep vein thrombosis or pulmonary embolism, should exercise extreme caution when considering dermal filler treatment.
A blood clot in the deep veins of the legs, known as deep vein thrombosis (DVT), can be life-threatening if it breaks loose and travels to the lungs, causing a blockage that cuts off oxygen from reaching the heart, lungs, or brain. This is known as a pulmonary embolism (PE).
Individuals with a history of DVT or PE are at increased risk of developing another blood clot in the future.
Dermal fillers, such as hyaluronic acid, calcium hydroxylapatite, and poly-L-lactic acid, can pose a significant risk to individuals with a history of blood clots.
The main concern is that the filler material may cause a sudden increase in pressure within the affected vein, which could dislodge a blood clot and lead to another pulmonary embolism.
Furthermore, dermal fillers can also affect blood flow and increase the risk of thrombosis.
In particular, individuals with a history of DVT or PE should avoid fillers that contain lidocaine, such as Juvederm Ultra, which contains the local anesthetic.
The American Society for Dermatologic Surgery recommends that patients with a history of DVT or PE exercise extreme caution when considering dermal filler treatment and should consult their doctor before undergoing any procedure.
Additionally, it is also recommended that individuals with a history of blood clots inform their healthcare provider about all medications, including over-the-counter supplements and herbal remedies, before undergoing dermal filler treatment.
This information can help identify potential interactions between the filler material and medications that may increase the risk of bleeding or thrombosis.
Ultimately, individuals with a history of blood clots should consult their doctor to discuss the risks and benefits of dermal fillers and determine if they are safe for use in their individual case.
The consultation will help healthcare professionals weigh the potential benefits of dermal fillers against the potential risks and develop a personalized treatment plan that prioritizes the patient’s health and well-being.
To minimize potential risks, individuals with certain medical conditions must exercise caution before undergoing cosmetic procedures that may increase the risk of blood clots.
In patients with a history of deep vein thrombosis (DVT) or pulmonary embolism, it’s recommended to avoid fillers altogether until the condition has been fully treated and stabilized.
Those with bleeding disorders such as hemophilia or platelet function disorders should also approach filler procedures with caution, as they may be at increased risk of excessive bleeding or bruising.
Individuals with blood clotting conditions like antithrombin deficiency, protein C deficiency, or factor V Leiden mutation should consult their healthcare provider before undergoing filler treatments.
Patients with active infections, such as cellulitis or abscesses, may be at increased risk of bleeding complications and should wait until their condition has been fully treated before receiving fillers.
Those with a history of blood clots or stroke should exercise extra caution when considering filler procedures, especially if they have a history of cardiovascular disease or are taking anticoagulant medications.
In addition to these conditions, individuals with liver disease, kidney disease, or autoimmune disorders such as rheumatoid arthritis or lupus may need to take special precautions when undergoing filler treatments.
Asthma, chronic obstructive pulmonary disease (COPD), and other lung conditions should also be carefully evaluated before filler procedures, as these conditions can increase the risk of complications.
Women with active cancer or those who are pregnant or breastfeeding should consult their healthcare provider before undergoing filler treatments, as the risks associated with these conditions may interact with the benefits of fillers.
It’s also essential to note that certain medications, such as anticoagulants, nonsteroidal anti-inflammatory drugs (NSAIDs), and blood thinners, can increase the risk of bleeding complications when combined with filler treatments.
Individuals with a history of adverse reactions to anesthetics or local anesthetics used in filler procedures should exercise caution and discuss their medical history with their healthcare provider before proceeding.
In some cases, it may be necessary for patients with certain medical conditions to undergo testing or evaluation before receiving fillers, such as blood clotting studies or imaging tests to evaluate cardiovascular risk.
By understanding the potential risks associated with filler procedures in individuals with pre-existing medical conditions, patients and their healthcare providers can make informed decisions about safe and effective treatment options.
Pregnancy and Breastfeeding (continued)
Pregnant women are generally not recommended to use fillers, as they can pose a risk to the developing fetus.
The risks associated with using fillers during pregnancy include the transmission of infectious diseases, such as HIV and hepatitis B, through contaminated needles or syringes.
Additionally, some fillers may contain preservatives that are not compatible with the medications used during pregnancy, which can lead to adverse effects on the fetus.
In particular, women who are using estrogen-containing fillers are at higher risk for complications during pregnancy and childbirth, including gestational diabetes and hypertension.
Women who have received fillers containing human dexamethasone (Deca-Durabolin) may also be at increased risk for preterm labor and birth defects, particularly if they become pregnant while still taking the medication.
Breastfeeding women are generally advised to avoid using fillers that contain local anesthetics, such as lidocaine or benzocaine, as these can pass into breast milk and potentially harm the baby.
However, some breast cancer patients may be prescribed fillers containing trastuzumab (Herceptin) to treat breast cancer-related pain, in which case the benefits of treatment may outweigh the risks during breastfeeding.
The American College of Obstetricians and Gynecologists (ACOG) and the Food and Drug Administration (FDA) provide guidelines for women who are pregnant or breastfeeding and considering cosmetic treatments that involve fillers.
These organizations emphasize that each individual case should be evaluated on its own merits, taking into account factors such as the type of filler used, the patient’s medical history, and any potential interactions with other medications or underlying health conditions.
The FDA requires manufacturers to conduct clinical trials to ensure safety during pregnancy and lactation before approving fillers for use in these populations.
Additionally, many countries have laws and regulations governing the sale and administration of cosmetic treatments to pregnant and breastfeeding women, such as restrictions on the use of certain medications or fillers.
In summary, while some fillers may be safe during pregnancy and breastfeeding under close medical supervision, they should not be used without consulting a healthcare provider first, and individuals should carefully weigh the potential benefits against the risks before making an informed decision.
Certain individuals may want to exercise caution when considering fillers due to their unique medical needs or medications they are currently taking.
Pregnant women, for example, face a range of considerations when it comes to fillers. The American College of Obstetricians and Gynecologists (ACOG) advises that fillers should generally be avoided during pregnancy, unless absolutely necessary.
There is limited research on the safety of fillers in pregnant women, but the available data suggest potential risks associated with filler use during this critical period.
One major concern is the transfer of foreign substances to the fetus. Filler materials are not completely biocompatible and can potentially migrate across the placenta if used near term or in early pregnancy.
Additionally, some fillers may stimulate the uterine muscle, which could potentially disrupt an already delicate pregnancy balance.
Another group of individuals who may need to be cautious with filler use are those on certain medications. For instance, blood thinners can increase the risk of bleeding complications if used in conjunction with fillers.
Corticosteroids and immunosuppressants also present unique challenges when considering filler use. These medications can suppress the immune system, potentially interfering with the body’s ability to react to foreign substances.
Furthermore, corticosteroids have been linked to changes in fat metabolism and distribution, which could impact the effectiveness and longevity of fillers.
Immunosuppressants, on the other hand, can weaken the immune system, making it more susceptible to infection or allergic reactions from the filler material.
Medications like beta-blockers, certain antidepressants, and anticonvulsants may also interact with fillers or increase their absorption rates.
It is crucial for individuals taking any of these medications to discuss their treatment options with their doctor before considering fillers.
Closely monitoring the body’s response to filler materials in these individuals can help mitigate potential complications and ensure a safer outcome.
Ultimately, a comprehensive evaluation by a qualified healthcare professional is essential for determining whether fillers are suitable for an individual with unique medical needs or medications.
Pregnancy and breastfeeding pose unique challenges for women with underlying medical conditions, requiring careful consideration to ensure a safe and healthy experience.
Women with certain medical conditions, such as diabetes, hypertension, or thyroid disorders, may be advised to exercise extra caution when using certain fillers during pregnancy or breastfeeding.
For instance, women with diabetes should inform their healthcare provider about the type of filler they plan to use, as some fillers contain ingredients that can affect blood sugar levels or insulin sensitivity.
Similarly, women with hypertension may need to monitor their blood pressure more closely when using fillers, as certain ingredients in the filler can cause blood vessels to constrict and increase blood pressure.
Thyroid disorders also require careful consideration. Women with hypothyroidism should avoid fillers that contain iodine, as excessive iodine consumption can exacerbate thyroid problems.
Furthermore, women with autoimmune disorders such as rheumatoid arthritis or lupus may be more susceptible to adverse reactions from fillers, and should discuss their specific concerns with their healthcare provider.
Asthma and allergies also require attention when using fillers. Women with a history of asthma may experience increased airway constriction or inflammation after receiving certain fillers, while women with allergies may react to ingredients in the filler.
Women with bleeding disorders, such as hemophilia or von Willebrand disease, should take special precautions when undergoing cosmetic procedures involving fillers, as the procedure can increase the risk of bleeding.
Certain medications that pregnant or breastfeeding women are taking may interact with fillers and lead to adverse effects. Women taking anticoagulant medications, such as warfarin, for example, may need to adjust their medication regimens when using fillers.
Additionally, women with certain heart conditions, such as arrhythmias or coronary artery disease, may require closer monitoring of their cardiovascular health when using fillers.
The American College of Obstetricians and Gynecologists (ACOG) and the American Academy of Dermatology (AAD) recommend that pregnant or breastfeeding women discuss their specific medical condition with their healthcare provider before undergoing any cosmetic procedure involving fillers.
This discussion will help ensure that the risks associated with using fillers are carefully weighed against the benefits, and that alternative options are explored if necessary.
Ultimately, a woman’s underlying medical conditions should be taken into account when deciding whether to use fillers during pregnancy or breastfeeding, and her healthcare provider should be involved in this decision-making process.
No one under 22 years old should undergo filler procedures.
Pregnant or breastfeeding women should be advised not to use facial fillers until after they have finished breastfeeding, as the effects of the fillers on a fetus or baby are unknown.
Patients with certain medical conditions may need to avoid filler procedures or take special precautions. For example:
No one with active skin infections such as acne, chicken pox, or herpes should use facial fillers.
No one with cold sores should use facial fillers until the sores have healed.
No one taking certain medications, such as blood thinners or antibiotics, should use facial fillers without consulting their doctor first.
No one with a history of bleeding disorders, such as hemophilia, should use facial fillers without consulting their doctor first.
No one taking immunosuppressive medications, such as corticosteroids, should use facial fillers without consulting their doctor first.
Patients with kidney disease may need to avoid filler procedures or take special precautions. For example:
The risk of allergic reaction to fillers is higher in people with kidney problems.
No one with liver disease should undergo filler procedures until their condition has been brought under control.
Pregnancy can affect the way your body reacts to fillers, so patients who are pregnant or trying to get pregnant may want to wait until after they have finished breastfeeding before having filler procedures.
Women over 40 years old may be at increased risk of certain complications from filler procedures. For example:
No one over 65 years old should undergo filler procedures.
Patients with diabetes may need to avoid filler procedures or take special precautions, as high blood sugar levels can increase the risk of complications.
People taking immunosuppressive medications, such as corticosteroids, may be at increased risk of certain complications from filler procedures.
No one should have a filler procedure if they are on long-term warfarin therapy (blood thinner) without discussing it with their doctor first.
Patients with active cancer, bleeding disorders or taking high doses of aspirin should not undergo filler procedures until they have stopped the treatment.
Read more about N City Magazine here. Read more about Josie Barrett here. Read more about Melissa Neufeld here. Read more about Plinr here.
- Upper Face Anti Wrinkle Treatment Near Burstow, Surrey - January 5, 2025
- Skin Pen Microneedling Near Mitcham, Surrey - January 3, 2025
- Pyrophilia Fetish: The Erotic Fascination With Flames - January 2, 2025

